
According to classical electromagnetic theory, only continuous spectra should be observed. The origin of discrete spectra in atoms and molecules was extremely puzzling to scientists in the late nineteenth century. Passing the light through a prism produces a line spectrum, indicating that this light is composed of photons of four visible wavelengths. For example, when an electric discharge passes through a tube containing hydrogen gas at low pressure, the H 2 molecules are broken apart into separate H atoms and a blue-pink color is observed. Each element displays its own characteristic set of lines, as do molecules, although their spectra are generally much more complicated.Įach emission line consists of a single wavelength of light, which implies that the light emitted by a gas consists of a set of discrete energies. Fluorescent light bulbs and neon signs operate in this way. Exciting a gas at low partial pressure using an electrical current, or heating it, will produce line spectra. In contrast to continuous spectra, light can also occur as discrete or line spectra having very narrow linewidths interspersed throughout the spectral regions.
#Atomic emission spectrum of hydrogen series#
Photons produced in this manner have a range of energies, and thereby produce a continuous spectrum in which an unbroken series of wavelengths is present. When solids, liquids, or condensed gases are heated sufficiently, they radiate some of the excess energy as light.
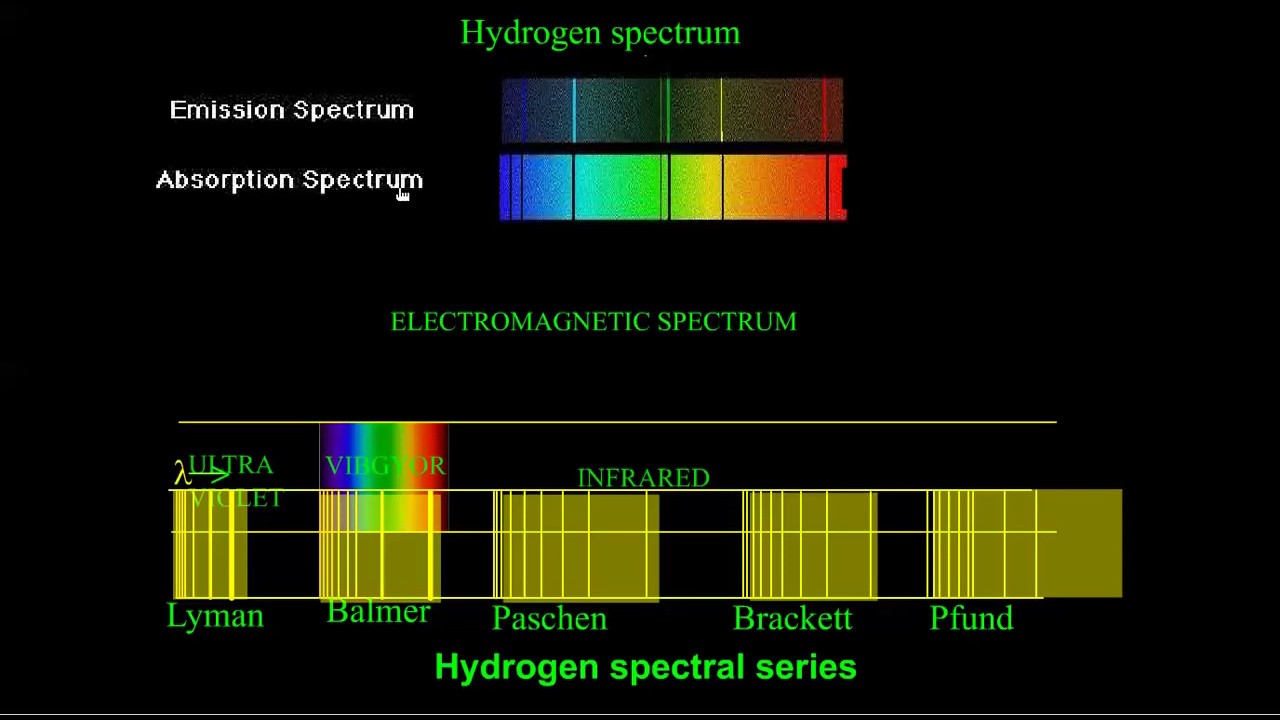
These are the wavelengths of light that are absorbed by a hydrogen atom when it is exposed to a continuous white light spectrum. Looking at hydrogen, the lines in its absorption spectrum are located at the same wavelengths of its emission spectrum, but they are dark. The inverse of an emission spectrum is its absorption spectrum. Since different atoms have different energy levels, the spectral emission lines vary from element to element and are used to identify substances.

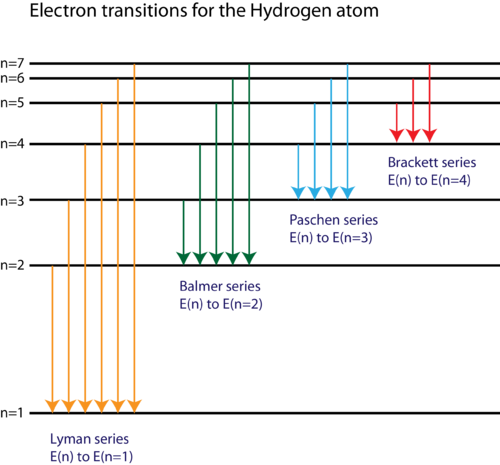
The wavelengths of spectral lines for hydrogen can be predicted using a mathematical expression, where R H is the Rydberg constant, n 1 is the principal quantum number of the lower energy level, and n 2 is the principal quantum number for the higher energy level. Additional spectral lines can be measured outside of the visible range, such as the Lyman series in the UV region and the Paschen series in the infrared region. The visible light spectrum appears as spectral lines at 410, 434, 486, and 656 nm, which correspond to energy level transitions from n = 3, 4, 5, and 6, respectively, to n = 2. It occurs when electrons transition from an energy level higher than n = 3 back down to n = 2. The set of spectral lines in the visible light region is known as the Balmer series. This is the emission spectrum for hydrogen. With pure elemental species, the emission behavior appears as lines of specific wavelengths rather than a broad spectrum. High energy emitted light results from electrons relaxing from a higher energy level, and low energy emitted light results from electrons relaxing from a lower energy level.Īn emission spectrum is a measure of emitted radiation across a range of wavelengths. The wavelength of the absorbed and emitted light depends on the difference between the high and low energy levels.
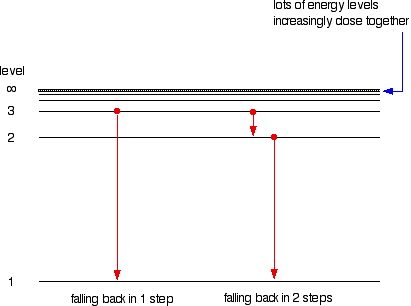
As the electrons relax down to either a lower energy state or to the ground state, the excess energy is released as a photon. from Wikipedia.īy contrast, if the detector sees photons emitted directly from a glowing gas, then the detector often sees photons emitted in a narrow frequency range by quantum emission processes in atoms in the hot gas, resulting in an emission line.When an atom absorbs energy, the electrons become excited and move to a higher energy level. Dips are present at the Fraunhofer line wavelengths.


 0 kommentar(er)
0 kommentar(er)
